Here is how we work
LEAD
Manage executive level directives for FDA\regulatory and commercialization strategy

STRATEGY
Convert passive industry engagement with academia into active, mutually beneficial and sustainable pipelines
PLAN
Accelerate commercialization initiatives with innovative evidence driven strategies

EXECUTE
Create win-win opportunity for institutional external exposure from engagement with commercial enterprises
Services Offered
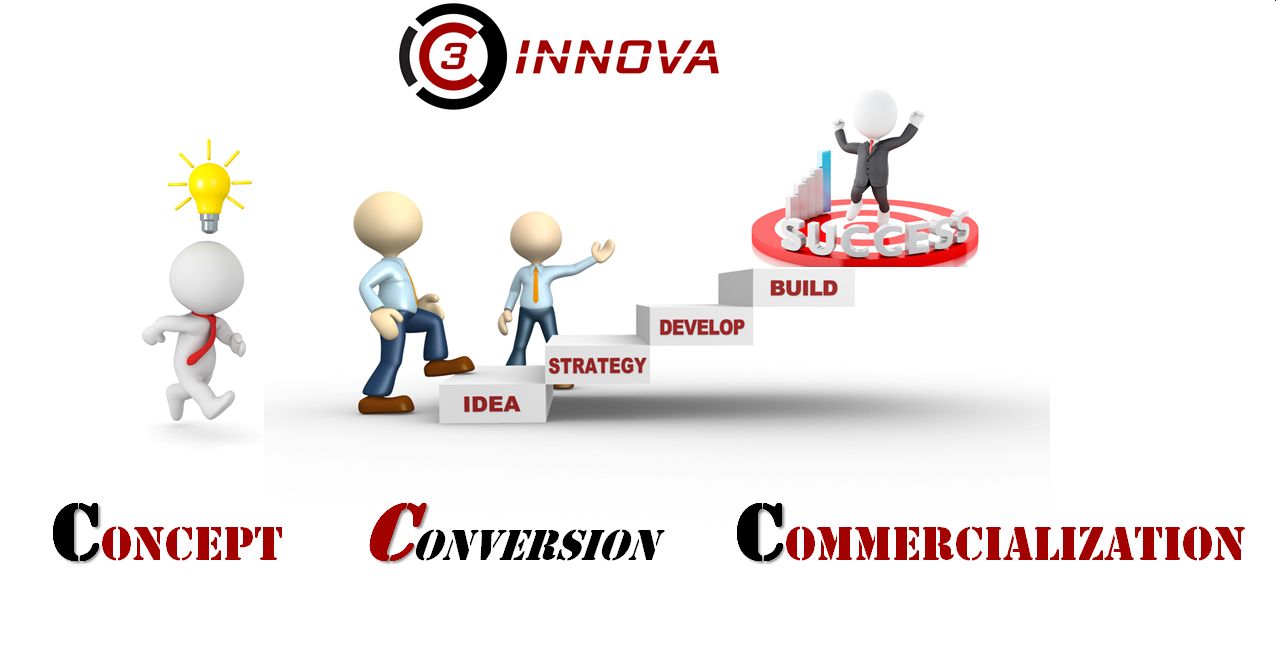
C3 Innova serves to accelerate commercialization progress through our ability to translate information between various disciplines and implement the necessary steps from concept to defendable claims in the market.
● Introductory communication and negotiation support for academic engagement ● Advocate nimble and marketable approaches that incentivize industry engagement ● IP evaluation services ● Scientific Premise ● Early stage evidence assessment ● Appraisal of Concept to clinic potential ● Clinical Study Design ● Streamlined negotiation structure and adapt to innovative boundaries of engagement ● Proven contract negotiations strategies ● IP/data commodity consideration and monetization ● Structured adaptability to achieve time sensitive opportunities ● Strategies for clinical trial translation from science ● Identification and engagement of key opinion leaders ● Linkages for global research opportunities to enhance worldwide adoption ● Procurement of non-dilutive funding ● Support with development and procurement of extramural grants and contracts ● Scientific interpretation of investigation results ● Support with FDA/regulatory reporting,
Contact us if we can help you

About
C3 Innova
“C3 Innova uniquely straddles integral commercialization relationships to accelerate concept transition to the marketplace.”
CONTACT
Get in touch
with us
info@c3innova.com








